– Policy Brief
Summary of request/problem
The duration of protection against COVID-19 after vaccination and infection needs to be assessed in order to adjust the duration of a vaccination/convalescence certificate and estimate the need for revaccination.
Executive summary:
Recent studies have characterized the decay kinetics of SARS-CoV-2 specific B and T cell responses after vaccination or infection. They estimate that neutralizing antibody IgG titers decrease with a half-life of around 100 days, while T cell responses seem to be longer lived with a half-life or around 150 days. Although a true immunologic correlate of protection from SARS-CoV-2 infection has not yet been identified, recent studies estimate based on SARS-CoV-2 Spike specific IgG levels and efficacy of different vaccines as well as protection levels from re-infection in covalescents that 20% of the initial convalescent antibody titer seems to be necessary for 50% protection from re-infections with mild to moderate symptoms and 3% to protect by 50% from severe re-infections. After natural infection, protection from mild reinfection might last at least 8 months and protection from severe disease about 16 months. In people >65 years, this may be shortened to 3-6 months and 10-12 months, respectively. After mRNA-vaccination, 2-4 fold higher initial neutralizing antibody titers are reached than after SARS-CoV-2 infection. In the young, this could prolong protection against mild infection to 16 months and against severe infection beyond three years. However, in the people >70-75 years, protection against mild infection may only last for 7-10 months and against severe infection for 15-24 months. These preliminary estimates rely on several assumptions and extrapolations of existing data from other vaccines leading to substantial uncertainty. Most importantly, partial immune escape variants and variants with enhanced transmission circulating in different regions globally may become dominant in Switzerland within the next months. Protection against such variants is predicted to be significantly lower and shorter lived. Therefore, sequencing of circulating viral variants should be accompanied by longitudinal immunological monitoring of risk groups for premature immune attrition predictive for susceptibility of severe disease. If prevention of severe disease remains the primary goal of the overall vaccination strategy, most individuals are unlikely to require booster doses within 18-24 months. However, people >70-75 years and other risk populations may profit from booster immunizations within one year of primary immunization and before expected epidemiologic acceleration in winter. If the strategy is expanded to limit (variant) virus circulation in the winter months, the target of primary immunization coverage needs to be increased to >80-85% of the adult population and booster doses will likely be necessary before 2022 to maintain a sufficient level of population immunity.
Main text
Recent studies on COVID vaccine induced (mRNA-1273, NVX-CoV2373, BNT162b2, rAd26-S+rAd5-S, ChAdOx1 nCoV-19, Ad26.COV2.S and CoronaVac) immune responses and in COVID convalescents have provided insights into the half-life of different SARS-CoV-2 specific immune compartments. Together with vaccine efficacy and reinfection data and modelling, the duration of protection from (re-)infection with mild to moderate disease symptoms paralleling transmission ability versus (re-)infection causing severe disease has been estimated. We will summarize some of this information in this document.
1. Half-life of SARS-CoV-2 specific immune responses
On the basis of neutralizing antibody responses similar decay kinetics for neutralizing antibody titers have been observed for COVID-19 convalescents and vaccine recipients 1-3. From these and other studies a picture emerges (Figure 14) that the half-life of IgG neutralizing antibody titers measured in the blood is around 100 days during the initial 250 days after infection or vaccination, while SARS-CoV-2 Spike specific IgM and IgA antibody titers have shorter half-lifes below 60 days. A very recent study measuring receptor-binding domain (RBD) specific IgG for one year after infection suggested a similar half-life of 100-120 days for the initial 6-8 months after SARS-CoV-2 infection with a stabilization thereafter5. SARS-CoV-2 Nucleoprotein specific IgG antibodies with presumably more limited or no protective function have a half-life between 60 and 90 days. Accordingly, SARS-CoV-2 specific plasma cells could still be detected in the bone marrow of convalescents 7 to 8 months after infection 6. In contrast to this decay of circulating antibody titers, SARS-CoV-2 specific memory B cells accumulate in frequency during the first six months after infection and continue to accumulate somatic hypermutations in their immunoglobulin loci for more potent neutralizing antibody production after restimulation 2,7. From these memory B cells, protective immune responses will likely be re-stimulated within 5-8 days after re-infection (or booster vaccination) as observed during single dose vaccination of COVID-19 convalescents8.
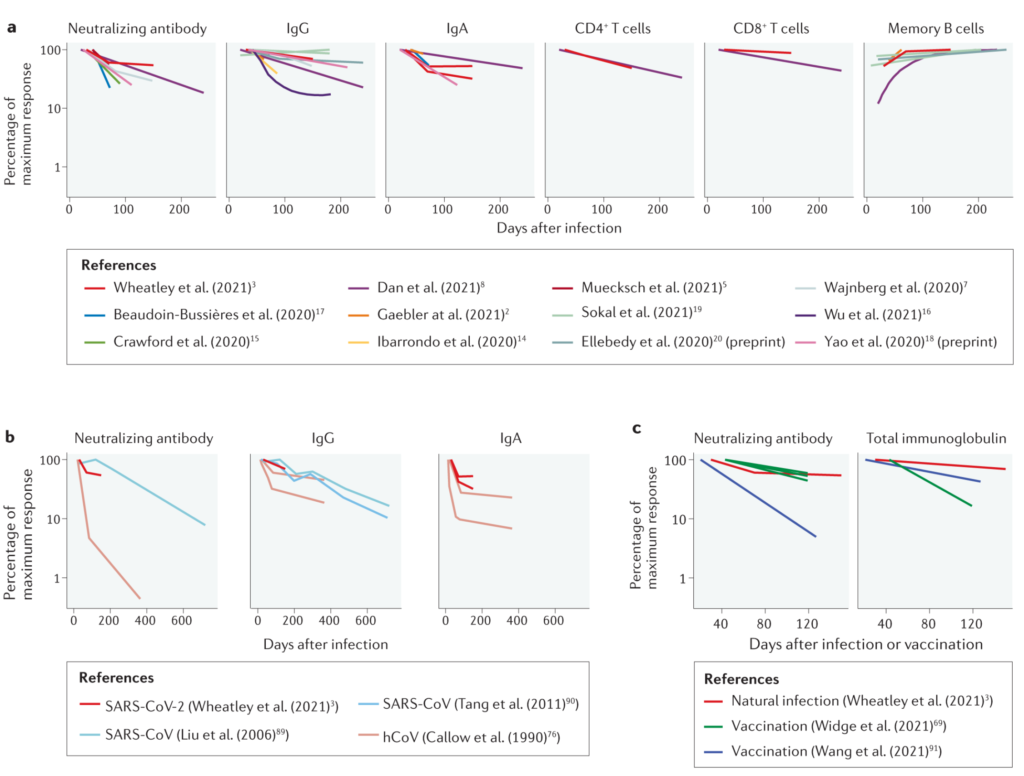
Figure 1. The decay of immune memory to coronavirus infections (Figure 1 from Cromer et al. 20214).

In addition to antibody responses, T cell responses can be detected in convalescents that focus on the recognition of Spike, Membrane, Nucleoprotein, nsp3 and ORF3a of SARS-CoV-2 3,9. Spike, Membrane, Nucleoprotein and ORF3a specific CD8+ T cell responses decay with a half-life of 120-190 days 2,3, and Spike, Membrane, Nucleoprotein, ORF3a and nsp3 specific CD4+ T cell responses slightly faster with a half-life of 140-150 days 2,3. These responses complement B cell responses in preventing severe disease after SARS-CoV-2 reinfection, but most likely cannot prevent SARS-CoV-2 infection and transmission as effectively as (neutralizing) antibody responses.
2. Estimated kinetics of protection against re-infection with mild or severe symptoms
Although a true correlate of protection from SARS-CoV-2 infection after vaccination or after recovering from a first infection has not yet been established, it is highly likely that neutralizing IgG are substantially involved. By correlating the neutralizing antibody titers after vaccination or after SARS-CoV-2 infection with (re-)infection outcomes, the level of antibody titers associated with 50% protection from asymptomatic/mild infection versus severe infection were estimated and compared to SARS-CoV-2 naïve and unvaccinated individuals, respectively 1. Khoury et al propose that a level of 20% of the initial average antibody titer after convalescence is associated with 50% protection against asymptomatic/mild infection and 3% of the initial titer is required for 50% protection against severe infection1. The respective study has a number of caveats that are also acknowledged by the authors. Namely, neutralizing antibody titers are used as protective correlate, while some individuals might clear SARS-CoV-2 infection primarily with T cell responses. Furthermore, neutralizing antibody responses were measured with different assays in the included studies. Thirdly, classification into re-infection with mild to moderate or severe symptoms uses different clinical symptoms in the analyzed studies. Fourthly, starting titers after vaccination might be higher after vaccination than after infection (2 to 4 fold10) and lower after convalescence of individuals with more advanced age if stratified according to disease severity 10,11. Finally, SARS-CoV-2 variants with a relevant transmission advantage and/or partial antibody immune escape phenotype might infect vaccinees or convalescents at higher antibody levels and thus earlier after the initial infection episode or immunization 12-14. Nevertheless, with keeping these confounding factors in mind, the antibody response to SARS-CoV-2 might decline to 20% of the initial level associated with 50% protection against mildly symptomatic re-infection with possible transmission after 240 days (8 months) in individuals <65 years of age. In covalescents above 65 years of age, protection against mild re-infection was shown to be more short-lived and dropped 50% within 3-6 months 10. Assuming an antibody decline with a stable half-life of 100 days for 1.5 years reaching a steady-state with a half-life of >10 years thereafter 1, the threshold of 3% of the initial antibody titer associated with 50% protection against severe infection would be reached in convalescents <65 years of age after 16 to 18 months and in those above 65 years after 10 to 12 months, unless natural virus circulation or vaccination would boost immunity before.
3. Estimated kinetics of mRNA-vaccine induced protection against infection with mild or severe symptoms
After vaccination with highly immunogenic mRNA vaccines initial neutralizing antibody titers are 2-4-fold higher than after natural infection and short-term protective immunity is at 95% in those populations enrolled in phase 2/3 clinical trials15. Population data from Israel have demonstrated short term BNT/Pfizer vaccine effectiveness of 94% against any SARS-CoV-2 infection and of 97% against severe infection also in people >85 years of age 16. Only for asymptomatic SARS-CoV-2 infection, there was a trend of reduced vaccine effectiveness according to age (83% in >85 years; 88% in 65-84 years; 91% in 45-64 years; 94% in 16-45 years). Together with recent data on some age dependence of vaccine induced neutralizing IgG titers17 this indicates that induction of protective immunity after mRNA COVID-19 vaccines is reduced in older individuals above 70-75 years of age although this seems to be much less pronounced than for other vaccines like influenza 18. Moreover, data on mid-term maintenance of protection of convalescents against SARS-CoV-2 re-infections indicate some age dependence as well: Above 65 years of age, protection decayed to 47% within 3-7 months whereas younger individuals maintained protection at >80% 10. Therefore, it is very likely that potential protective thresholds against severe COVID-19 are reached at earlier time points in people >70-75 years of age not only after recovering from natural infection but also after mRNA vaccination. According to the modelling of Khoury et al., individuals below 65 years of age should maintain >50% protection against mild infection for 500 days (16 months) or even longer after two doses of mRNA vaccines (Figure 3c, lower panels, starting efficacy 90-95% 1) and against severe infection at >80% for more than 3 years as long as circulating virus variants do not exhibit significant antibody immune escape phenotypes. For severe infection outcomes we anticipate that a potential protective threshold of 50% is not sufficient from a public health perspective, since 50% of infected individuals would be at risk for hospital admission despite vaccination. We therefore extrapolated the data of Khoury et al. to provide assumptions of 70-80% protection for severe outcomes 1. Assuming that antibody decay kinetics are largely age independent, the situation is likely to be different and comparable to starting efficacies of 80-90% in people >70-75 years of age (Figure 3c1). Protection against mild infection would fall below the 50%-threshold within 200-300 days (7-10 months) and against severe infection below a 70% protection threshold within 450 to 750 days (15-24 months). However, after two doses of mRNA vaccines a 50% protection level against severe COVID-19 may also be maintained for more than 3 years in the elderly and very elderly. Therefore, the level of desired protective immunity will dictate at which time point vaccine booster doses may be required as long as natural virus circulation is very limited and circulation of (partial) immune escape variants does not warrant for earlier boosts with adequately adapted vaccines.
Specifically, if circulating virus variants exhibit significant antibody immune escape phenotypes the initial level of protection and its duration will be substantially reduced. For a 5-fold decreased potency for variant neutralization as estimated for B.1.617.2 (or delta variant)17, efficacy of mRNA vaccines starts at 77% instead of 95% protection and the 20% neutralizing capacity associated with 50% protection against mild to moderate infection is already reached after 200 days1. More importantly, the threshold of 70% protection against severe infection may be reached within 300-500 days (10-18 months) in individuals <65 years age and even earlier those above 70-75 years. In this case, booster vaccinations with adapted mRNA vaccines containing the Spike protein sequence of relevant variants should be considered within one year and before anticipated epidemiologic acceleration during the cold season. Booster vaccinations with variant sequences are likely to induce neutralizing antibodies both against the variant and the original virus as shown after natural SARS-CoV-2 infection, where convalescent sera from patients infected with the immune escape variant B.1.351 contained broadly neutralizing IgG with comparable activity against B.1.351, the original virus and other SARS-CoV-2 variants19.
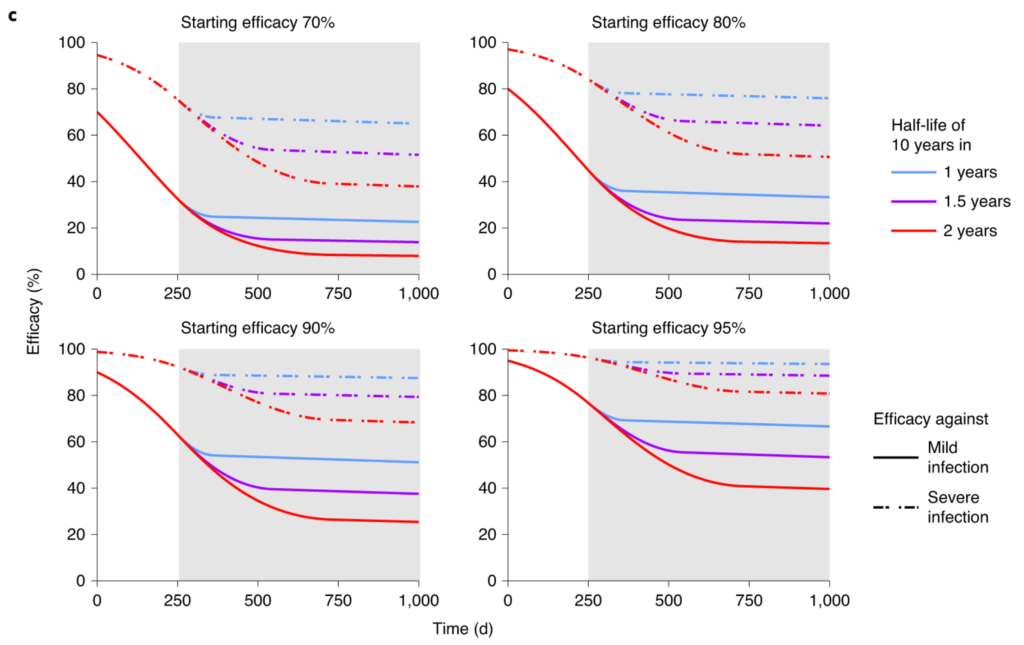
Figure 2. Protection from severe infection (Figure 3c from Khoury et al. 2021 1)
[…] We simulate three scenarios, with decay of neutralization taking 1 year (blue dashed line), 1.5 years (purple dashed line) or 2 years (red dashed line) to slow to a 10-year half-life. For different initial starting levels the model projects the decay in neutralization titer over the subsequent 1,000 d (the gray shaded area indicates projections beyond the currently available data). […] c. Extrapolation of the trajectory of protection for groups with different starting levels of protection. […] The projections beyond 250 d (gray shaded region) rely on an assumption of how the decay in SARS-CoV-2 neutralization titer will slow over time. In addition, the modeling projects only how decay in neutralization is predicted to affect protection. Other mechanisms of immune protection may play important roles in providing long-term protection that are not captured in this simulation
Conclusions
SARS-CoV-2 neutralizing antibody half-life is around 100 days during the initial 250 days of observation both after natural infection and after vaccination with likely stabilization thereafter. Nevertheless, extrapolations and modeling according to immune response kinetics after other vaccines were required to currently estimate the level and duration of protection against different outcomes of SARS-CoV-2 infection.
SARS-CoV-2 specific T cell half-life is around 150 days after natural infection.
Memory B and T cell responses are probably even more long-lived and memory B cells accumulate in the first 6 months after infection
After natural infection, neutralizing antibodies decay to a protective level of 50% against re-infection with mild to moderate symptoms within 8 months and to 50% protection against severe re-infection within 16 months in individuals below 65 years of age. In individuals >65 years, duration of protection is likely to be shorter: 3-6 months for 50% protection against mild infection and 10-12 months for 50% protection against severe infection.
After mRNA vaccination, individuals below 65 years of age likely maintain >50% protection against mild infection for 16 months or longer and >80% protection against severe infection for more than three years, whereas individuals above 70-75 years of age likely maintain >50% protection against mild infection for 7-10 months and >70% protection against severe infection for 15-24 months.
In case of circulation of immune escape variants with ≥5-fold higher antibody titers needed for neutralization, protective immunity may be reduced and shortened substantially with 50% protection against re-infection with mild to moderate symptoms and transmission after 200 days, and 70% protection against severe infection reached already within 10-18 months after vaccination also in those below 65 years of age.
The assessment of these antibody decay kinetics is mostly based on data from young and healthy vaccinees and extrapolated in time and beyond the covered age and risk groups. Solid data from people >70-75 years of age and from other risk groups are very limited. Therefore, the calculations provided here are very rough estimates of minimal duration of protection relying on several assumptions with inherent and substantial uncertainties. During the following months, it is crucial to generate and collect data on maintenance of immunity after infection and vaccination in different risk groups by detailed immune-monitoring. Some of these risk groups most likely start with a reduced protection level after vaccination or convalescence 10, and therefore will probably be at risk for (re-)infection earlier. Moreover, data on all individuals with proven vaccination failure or re-infection should be collected systematically in Switzerland. Besides thorough characterization of infecting virus variants these individuals need to be assessed clinically concerning severity of disease and immunologically concerning magnitude and breadth of humoral and cellular immunity.
It is expected that additional data related to immunogenicity and protective efficacy of mRNA vaccines in many diverse risk groups will become available internationally. Together with data from our own analyses, this will allow to evaluate more precisely which populations will profit from booster immunizations at which intervals. Regular updates of this report will be needed.
However, for the time being and with the primary aim to prevent severe COVID-19 booster immunizations for people >70-75 years of age and for other individuals at increased risk of severe COVID-19 may be required within one year of primary immunization and before epidemiologic acceleration during the cold season is to be expected. If deemed indicated, such COVID-19 booster doses may be synchronized pragmatically with the influenza vaccination campaign in autumn 2021 since the target populations largely overlap. If the vaccination strategy aims at much higher levels of population immunity capable to limit overall virus circulation, such booster immunizations need to be considered in general for the adult population to limit transmission in the coming winter season to parts of the population that are not eligible for vaccination, such as children younger than 12 years of age.
Concerning COVID-certificates, the current data on the level and duration of protective immunity suggest that for convalescents a certificate duration of 6 months seems appropriate. For persons fully vaccinated with mRNA vaccines (two doses or one dose in convalescents) an extension of certificate duration to 12 months seems scientifically justifiable.
References
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021.
- Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun. 2021;12(1):1162.
- Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529).
- Cromer D, Juno JA, Khoury D, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021.
- Li C, Yu D, Wu X, et al. Twelve-month specific IgG response 1 to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. bioRxiv. 2021;https://doi.org/10.1101/2021.04.05.437224.
- Turner JS, Kim W, Kalaidina E, et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021.
- Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639-644.
- Goel RR, Apostolidis SA, Painter MM, et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58).
- Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336-1345.
- Hansen CH, Michlmayr D, Gubbels SM, Molbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397(10280):1204-1212.
- Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18(2):318-327.
- Cavanaugh AM, Fortier S, Lewis P, et al. COVID-19 Outbreak Associated with a SARS-CoV-2 R.1 Lineage Variant in a Skilled Nursing Facility After Vaccination Program – Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):639-643.
- Thompson CN, Hughes S, Ngai S, et al. Rapid Emergence and Epidemiologic Characteristics of the SARS-CoV-2 B.1.526 Variant – New York City, New York, January 1-April 5, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(19):712-716.
- Vitale J, Mumoli N, Clerici P, et al. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Intern Med. 2021.
- Walsh EE, Frenck RW, Jr., Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439-2450.
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819-1829.
- Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021.
- Wagner A, Weinberger B. Vaccines to Prevent Infectious Diseases in the Older Population: Immunological Challenges and Future Perspectives. Front Immunol. 2020;11:717.
- Cele S, Gazy I, Jackson L, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593(7857):142-146.
See also the Policy Briefs Considerations for an alert system for infectious diseases, focus on COVID-19: a scoping review of tier systems used in other countries and current data / The role of children (≤12 years of age) and adolescents (13-17 years of age) in the SARS-CoV-2 pandemic: A rapid review
Type of document: Assessment of scientific evidence
In response to request from: NCS-TF
Date of request: 28/05/2021
Date of response: 10/06/2021
Experts involved: Expert Group Immunology
Contact persons: (members of the Expert Group Immunology): christian.muenz@uzh.ch, daniel.speiser@unil.ch, federica.sallusto@irb.usi.ch, manfred.kopf@biol.ethz.ch, urs.karrer@ksw.ch