– Policy Brief
Summary of request/problem
The duration of protection against COVID-19 after vaccination and infection needs to be assessed in order to adjust the duration of a vaccination/convalescence certificate and estimate the benefit of a three-dose vaccination scheme.
Executive summary:
People who have recovered from COVID-19 are protected from hospitalization at least as well as people who have received two doses of a mRNA vaccine. This evidence comes from studies that have characterized the magnitude, decay kinetics and protective capacity of SARS-CoV-2 specific B and T cell responses after vaccination or infection. Initial titers of neutralizing antibody titers are 2-4-fold higher after mRNA-vaccination than after SARS-CoV-2 infection. The similar levels of protection against hospitalization in vaccinated and recovered individuals suggests that additional immune responses, and possibly improved affinity maturation of SARS-CoV-2 Spike-specific antibody responses, compensate for overall higher SARS-CoV-2 Spike specific antibody responses in vaccinees. Furthermore, antibody titers vary at least 3-fold between individuals with severe and mild SARS-CoV-2 infection.
Protection against SARS-CoV-2 infection and associated hospitalization is waning both in people who have received two vaccine doses (double-vaccinated) and in those who have recovered from SARS-CoV-2 infection. Similarly, immune responses in vaccinees and convalescents are waning with similar kinetics. Studies comparing the three COVID vaccines that are licensed in Switzerland suggest that protection from mild breakthrough infection with the Delta Variant of SARS-CoV-2 decreases within 6 months from 85-90% to around 60% for mRNA-1273 (Moderna), to around 45% for BNT162b2 (BioNTech/Pfizer), and to around 20% for Ad26.COV2.S (Johnson&Johnson). SARS-CoV-2 infected BNT162b2 vaccinated individuals were also found to transmit the Delta variant of SARS-CoV-2 with 70% efficacy compared to naïve individuals 14 weeks after vaccination. Long term protection against mild reinfection after convalescence is more robust than after BNT162b2 and at least comparable to mRNA-1273 vaccination. Protection against severe symptoms after SARS-CoV-2 infection of vaccinees and convalescents is more robust and seems to have only declined to around 60-80% after 6 months of mRNA vaccination with similar risk factors predisposing for hospitalization as in unvaccinated individuals. This results primarily in significant hospitalization frequencies of vaccinated individuals among advanced aged groups and individuals with co-morbidities.
A third dose vaccination increases the protection against infection from round 50% to at least 95% across age classes. Third dose vaccination was shown to increase SARS-CoV-2 Spike specific antibody levels by more than 10-fold compared to the timepoint prior to the third dose application, and even 2- to more than 10-fold compared to antibody titers shortly after the second vaccine dose for both mRNA-1273 (Moderna) and BNT162b2 (BioNTech/Pfizer). SARS-CoV-2 breakthrough infection after two dose vaccination broadened immunity and demonstrated superior boosting of SARS-CoV-2 Spike specific antibody responses.
In older individuals (>65 years), a third dose of BNT162b2 (BioNTech/Pfizer) reduces the risk for both infection and hospitalization by more than 10-fold 14 days after third dose vaccination, but the durability of this response remains unclear. However, extrapolating the data on neutralizing antibody titers and waning protection after two dose mRNA vaccination it is expected that after a third dose protection against mild Delta infection and transmission should be maintained above 50% for 9-15 months also in risk groups like persons over 65 years of age.
Overall, a third dose has been shown in Israel to effectively prevent hospitalizations, especially in risk groups, as well as to limit virus transmission in the entire population (10-fold compared to double-vaccinated individuals) in the first months after boosting.
Main text
Recent studies on COVID-19 vaccines licensed in Switzerland, i.e. a two-dose schedule of the mRNA vaccines, mRNA-1273 (Moderna) and BNT162b2 (BioNTech/Pfizer), and a single dose of the viral vector vaccine, Ad26.COV2.S (Johnson&Johnson) have assessed the durability of their protective efficacy against SARS-CoV-2 infection, SARS-CoV-2 associated hospitalization, and prevention of SARS-CoV-2 transmission by vaccinees who have become infected. Here we summarize some of the current evidence about the duration of protection after vaccination and infection, as well as potential benefits of third dose vaccination.
1. Differences in SARS-CoV-2 specific immune responses after vaccination or infection
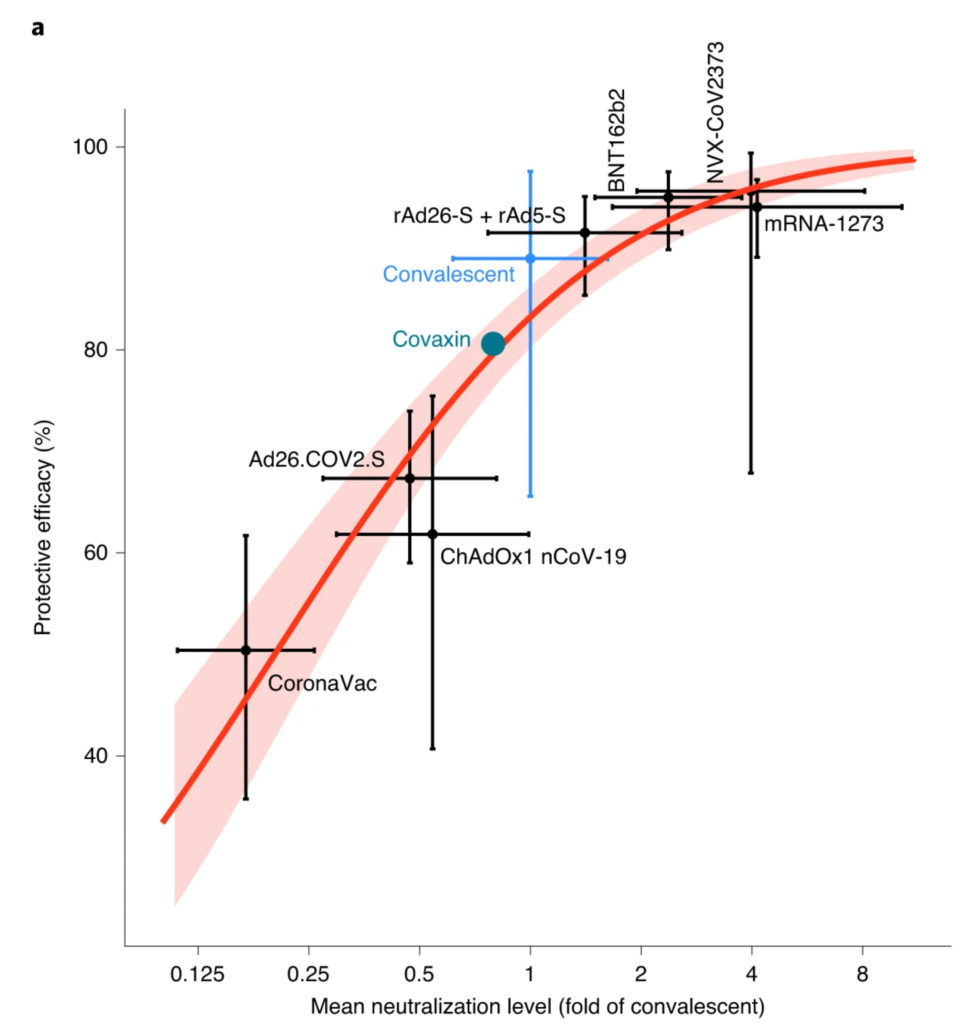
Figure 1: Understanding the relationship between neutralization and protection. The reported mean neutralization level from phase 1 and 2 trials and the protective efficacy from phase 3 trials for seven vaccines, as well as the protection observed in a seropositive convalescent cohort, are shown The 95% CIs are indicated as vertical and as horizontal whiskers. The red solid line indicates the best fit of the logistic regression model and the red shading indicates the 95% predictive interval of the model. (Figure 1a from Khoury et al., Nat Med 2021.)
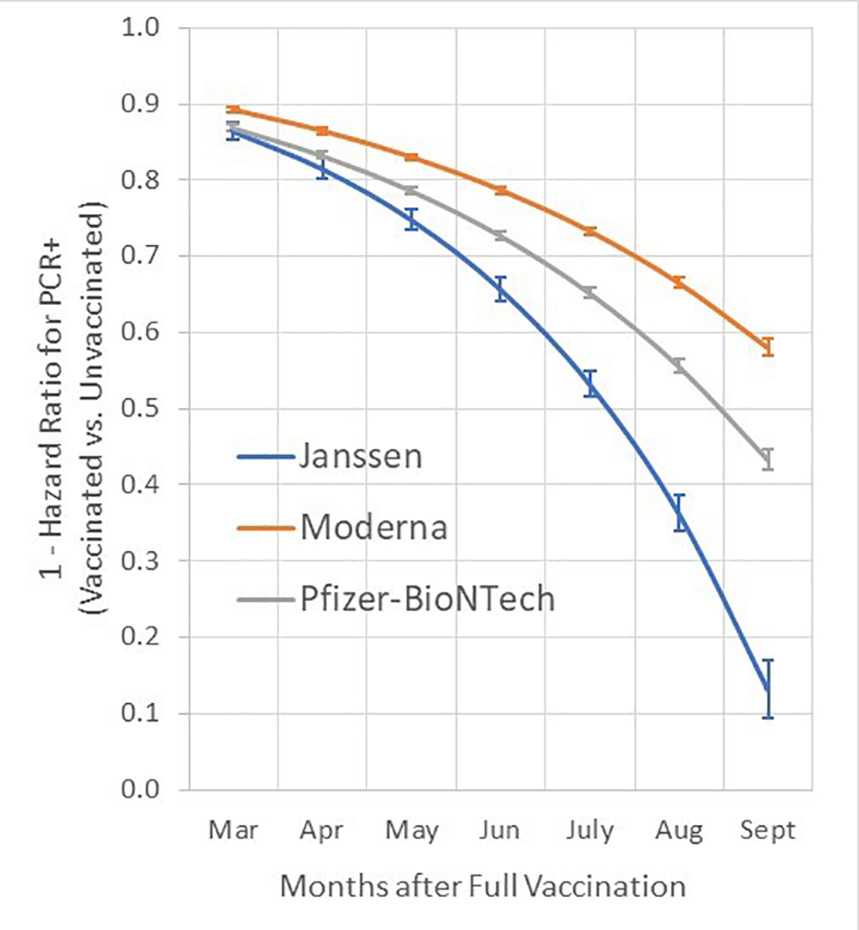
Figure 2: Time dependent vaccine effectiveness against SARS-CoV-2 infection as estimated from Cox proportional hazards models, adjusted for age, race, ethnicity, sex, and comorbidity score. Vaccine effectiveness presented as (1- hazard ratio * 100) and 95% confidence intervals. Effectiveness for each month was estimated from contasts using product terms for vaccination status by time to most recent RT-PCR assay.
(Figure 1 from Cohn et al., Science 2021.)
2. Kinetics of protection against re-infection with mild or severe symptoms
Virus neutralizing antibody levels were found to correlate with protection from SARS-CoV-2 infection in people who had received two doses of vaccine 4 (Figure 1). However, this protection wanes during six months after the second dose of vaccination for all three vaccines licensed in Switzerland. In a study from the US, protection from infection by SARS-CoV-2 decreased within 6 months from 85-95% to 60% for mRNA-1273, to 45% for BNT162b2, and to 15% for Ad26.COV2.S[13] (Figure 2). Similar decreases were also reported in the UK and Israel, among other countries[8],[14]–[18]. Reinfections with SARS-CoV-2 were also observed between 6 to 12 months after initial infection, although at a significantly lower rate than in BNT162b2 vaccinated individuals [19]. The best protection against reinfection was observed in individuals that had previously been infected and then been vaccinated by one dose of BNT162b2. Within a similar time scale SARS-CoV-2 infected BNT162b2 vaccinees regained the capacity to transmit the virus [15]. 14 weeks after the second dose, transmission capacity was found to be again 70% of the capacity of non-immune infected individuals (Figure 3). In contrast to a majority of vaccinees becoming susceptible to infection after 6 months, protection from severe disease and hospitalization was sustained at 80% after this time period, and mainly individuals with risk factors such as old age and comorbidities became susceptible to breakthrough infections with severe symptoms [14],[16],[18],[20],[21]. However, this protection further declined to below 50% between 6 and 9 months in a recent Swedish study including a substantial part of individuals immunized not with mRNA but adenovirus based vaccines [22]. Thus, breakthrough infection with mild symptoms and significant transmission capacity occurs in many vaccinees after six months (around 2-fold protection compared to unvaccinated), while protection from severe disease is still maintained at a high level of 60-80% in the people at risk (up to 5-fold protection) and around 10-fold protection in the general population. However, in the aged population and in individuals with comorbidities this modest reduction in protective efficacy puts a significant number of individuals at increased risk for hospitalization[28].

Figure 3: Rate ratios for positive PCR tests in contacts by time since second vaccination in 426 index cases (panel A), delta variant, and vaccine type. Panel A compares the rate of positive PCR results in tested contacts, comparing the impact of index case vaccination status to an unvaccinated index case. The dashed horizontal line indicates the probability of a positive-PCR result in an unvaccinated case after contact with an unvaccinated index case. The shaded area indicates the 95% confidence interval. There was no evidence that fitting different rates by variant for the change in protection over weeks since second vaccine improved model fit.
(Figure 1a, right panel from Eyre et al., medRxiv 2021.)
3. Protection after third dose vaccination or boosting by infection
Third dose of both mRNA-1273 or BNT162b2 raises neutralizing antibody responses against SARS-CoV-2 more than 10-fold and this level exceeds even the SARS-CoV-2 Spike specific antibody levels after second dose vaccination by 2- to more than 10-fold, depending on the study [23]–[25] Compared to two doses of primary mRNA-immunization 6 months previously this translates into a more than 10-fold increased protection against infection and hospitalization in the 65 plus year old population 14 days after a BNT162b2 third dose [26]. Population data from Israel suggest that a third dose 6 months after BNT162b2 will significantly decrease the risk of hospitalization in all age groups above 40 years compared to vaccination with two doses [27]. A breakthrough infection of vaccinated individuals seems to boost virus neutralizing antibody titers even higher, possibly 5-fold more than a third dose of mRNA-1273 [12],[23],[24]. However, the long-term protection by a third dose vaccination still needs to be evaluated and solid data will probably emerge >6 months after initiation of the booster campaign in Israel. A rough estimate of the potential duration of protection after boosting can be made using the following data: levels of neutralizing antibodies after boosting compared to levels after the 2nd dose (2- to 10-fold higher) [23]–[25], level of protection in relation to the level of neutralizing antibodies [4], half-life of SARS-CoV-2 neutralizing IgG of 90-110 days [4], and decay of protection against mild breakthrough infection after the second mRNA-vaccine dose to about 50% within 6 months [13]. Using these data we estimate that after a third dose, protection against mild Delta infection and transmission should be maintained at a level of more than 50% for 9-15 months for the whole population. Protection against severe infection of >80% is expected to be more long lasting. However, if viral variants with enhanced properties of immune evasion and/or increased transmissibility compared to Delta should become prevalent in Switzerland the duration or the level of protection might be substantially attenuated. Nevertheless, the available data on SARS-CoV-2 specific immunity confirm the basic immunological knowledge that with each additional antigen exposure (by re-infection or booster vaccination), the immune response may become stronger and more long-lasting.
Conclusions
Protection against infection wanes in BNT162b2 and mRNA-1273 double-vaccinated people to around 50% after 6 months. These vaccinees with breakthrough infection transmit the Delta Variant of SARS-CoV-2 with more than 50% of the efficacy of unvaccinated individuals. Giving vaccinated or infected individuals a third dose of a mRNA vaccine will increase their protection against infection to >95% within two weeks (ie. 20-fold protection compared to an unvaccinated individual). From an epidemiological perspective, this increase in protection against infection has at least four consequences. First, it decreases the overall number of infections so that virus circulation in the population is slowed down. This can reduce the burden on the health care system, in particular in the cold season where transmission of SARS-CoV-2 is elevated. Second, people in close contact with individuals who cannot be vaccinated or are very vulnerable can minimize – through a third dose – the risk that they infect these individuals. Third, by reducing the number of infections, the risk of post-acute sequelae of COVID-19 is reduced since these sequela (“long COVID”) have been shown to also occur after breakthrough infections [28]–[30]. Finally, protection against severe disease has waned to around 60-80% in 65-year-old or older vaccinees. A third dose vaccination reduces their hospitalization risk by more than 10-fold. This benefit does not only restore immune protection to levels after the second vaccine dose, but increases immune responses even further and seems to induce sufficient immunity in vaccinees for which two doses were not sufficient for protection against SARS-CoV-2 infection.
While the individual benefit of boosters takes effect within 10-14 days, giving the current epidemiologic situation (mid-November 2021) with doubling times of infections of about two weeks, the epidemiological benefits are largely dependent on the speed at which a third dose will be delivered to a large proportion of the eligible population.
References
[1] Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 2020;21:1336-45.
[2] Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371.
[3] Wheatley AK, Juno JA, Wang JJ, et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat Commun 2021;12:1162.
[4] Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021.
[5] van Gils MJ, Lavell AHA, van der Straten K, et al. Four SARS-CoV-2 vaccines induce quantitatively different antibody responses against SARS-CoV-2 variants. MedRxiv 2021.
[6] Molodtsov IA, Kegeles E, Mitin AN, et al. SARS-CoV-2 specific T cells and antibodies in COVID-19 protection: a prospective study. medRxiv 2021.
[7] Ali AM, Ali KM, Fatah MH, Tawfeeq MH, Rostam HM. SARS-CoV-2 Reinfection in Patients Negative 1 for Immunoglobulin G Following Recovery from COVID-19. medrxiv 2021.
[8] Delbrück M, Hoehl S, Toptan T, et al. Characterization of the humoral immune response to BNT162b2 in elderly residents of long-term care facilities five to seven months after vaccination. medrxiv 2021.
[9] Cho A, Muecksch F, Schaefer-Babajew D, et al. Anti-SARS-CoV-2 receptor binding domain antibody evolution after mRNA vaccination. Nature 2021.
[10] Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639-44.
[11] Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021;595:426-31.
[12] COLLIER AY, BROWN CM, MCMAHAN K, et al. Immune Responses in Fully Vaccinated Individuals Following Breakthrough Infection with the SARS-CoV-2 Delta Variant in Provincetown, Massachusetts. medRxiv 2021.
[13] Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021:eabm0620.
[14] Goldberg Y, Mandel M, Bar-On YM, et al. Waning Immunity after the BNT162b2 Vaccine in Israel. N Engl J Med 2021.
[15] Eyre DW, Taylor D, Purver M, et al. The impact of SARS-CoV-2 vaccination on 1 Alpha & Delta variant transmission. medRxiv 2021.
[16] Rosenberg ES, Holtgrave DR, Dorabawila V, et al. New COVID-19 Cases and Hospitalizations Among Adults, by Vaccination Status – New York, May 3-July 25, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1306-11.
[17] Nanduri S, Pilishvili T, Derado G, et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant – National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1163-6.
[18] Puranik A, Lenehan PJ, Silvert E, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv 2021.
[19] Gazit S, Shlezinger R, Perez G, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv 2021.
[20] Self WH, Tenforde MW, Rhoads JP, et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions – United States, March-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1337-43.
[21] Tenforde MW, Self WH, Naioti EA, et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults – United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-62.
[22] Nordstrom P, Ballin M, Nordstrom A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet Reg Health Eur 2021:100249.
[23] Atmar RL, Lyke KE, Deming ME, et al. Heterologous SARS‐CoV‐2 Booster Vaccinations – Preliminary Report. MedRxiv 2021.
[24] Chu L, Montefiori D, Huang W, et al. Immune Memory Response After a Booster Injection of mRNA-1273 for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). medrxiv 2021.
[25] Romero-Ibarguengoitia ME, Rivera-Salinas D, Hernández-Ruíz YG, et al. Effect of the third dose of BNT162b2 vaccine in quantitative SARS-CoV-2 spike 1-2 IgG antibody titers in healthcare workers. MedRxiv 2021.
[26] Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021;385:1393-400.
[27] Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021.
[28] https://sciencetaskforce.ch/en/scientific-update-of-26-october-2021/
[29] Antonelli et al., Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. The Lancet Infectious Diseases 2021.
[30] Bergwerk et al., Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med 2021.
[31] Preprint: Taquet et al., Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. medRxiv 2021.
Type of document:Assessment of scientific evidence (update)
In response to request from: NCS-TF
Date of request: : 10/11/2021
Date of response: Shared with FOPH on 17/11/2021
Individuals involved: Christian Münz, Urs Karrer, Daniel Speiser, Manfred Kopf, Richard Neher, Nicola Low, and Tanja Stadler
Contact persons: christian.muenz@uzh.ch, urs.karrer@ksw.ch
Comment on planned updates: This is an update of the PB on the same topic that was published on the 25th of June 2021