14. Oktober 2020 – Policy Brief
Summary of request/problem
Overview about mask types
Protection Masks
Medical/surgical Masks
Community Masks
“Community masks” have made their appearance in Switzerland mainly in the context of the present pandemic. In the public discussion, “Community mask” is a broad concept that embraces essentially all non-professional (including non-medical) masks that are destined to protect the greater public from infection by means of source control. Community masks range from home-made cotton masks to variously sophisticated textile masks. This mask type, which has the advantage that it can be produced and supplied locally, has been introduced to the Swiss market since the beginning of the pandemic. With the aim to reduce the transmission of the virus in the community (source control) and to protect “community mask” buyers from low quality products, a minimum performance level was rapidly defined within Switzerland. These voluntary minimum requirements and specifications were summarized in a policy brief published by the Swiss National COVID-19 Science Task Force on 25 April 2020 (and actualized XX September 2020). That document was intended to be a guideline for textile manufacturers for the production of community masks of sufficient quality, offering minimum protection. The recommendation defined quantifable and measurable levels for the filtration efficiency for 1 µm particles, liquid splash resistance, the level of air permeability, as well as reusability after standard washing procedures.
Of note, these recommendations are not a standard, in the strict sense of the term. A “standard” is a carefully elaborated document that has, before it is finalized and used to certify products to be marketed, undergone a long process of tests and evaluations by a large number of laboratories, at least across one country, often across many countries (for example across the European continent for European standards). Such standards are legally binding. Present recommendations for community masks are neither a standard nor a document to be used for the certification of products. Still, the present recommendations can and should be used to define, now and within Switzerland, what is and what is not an acceptable mask to protect against the spread of SARS-CoV-2 in the greater public. Different private testing institutes like Testex or SQTS offer testing services to verify whether a given community mask fulfills the recommendations of both the past and present policy briefs on the topic. Labels emitted by such private institutions offer guidance to both industry and the public, even though they are not legally binding.
A number of countries other than Switzerland have published similar guidelines; it is thus important for testing institutions and manufacturers to refer to the guidelines and criteria that have been used for testing.
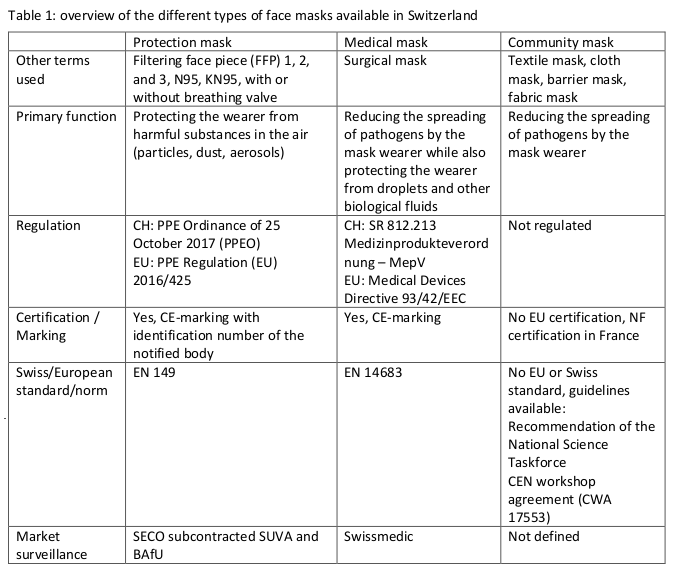
* for an air flow of 30 l/min; ** for particles sizes above 1 µm; *** for particles sizes above 3 µm; for detailed technical specification see also the revised Policy Breif of ‘Recommendation on minimal specification for CM and their use’ from 24.9.2020; nsctf, Swiss National Covid-19 Science Task Force; AFNOR, association française de normalisation; CWA, CEN workshop agreement
Architecture and composition of protection- and medical masks
Standards define performance criteria to be met before masks (of either type) can be put on the market. However, design and materials of the masks are not regulated; certain designs have proven their efficiency and are, therefore, often found in agreed products.
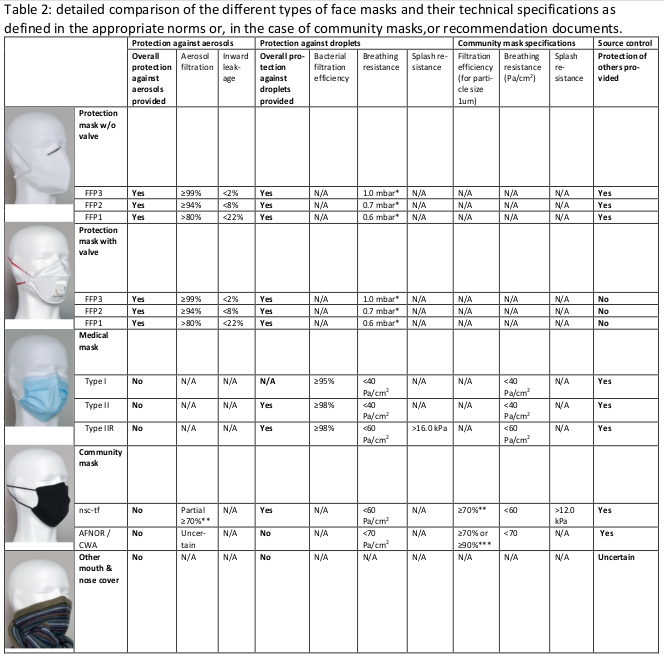
Usually, both protection masks and medical masks are made of synthetic fibers. The main filtration function is guaranteed by a thin layer of non-woven (i.e., randomly distributed within a plane) melt-blown polypropylene fibers. Those fibers are inexpensive, non-toxic and sufficiently fine and densely packed within the layer to block particles (particle of 1 micrometer or less) from passing. At the same time the fibers are packed so as to leave sufficient gaps for air to pass a rate comfortable for the wearer (this is expressed in Pa/cm2 in Table 2, the non-intuitive units come from the prescribed measurement procedure). Some protection masks additionally have a valve (see Table 2), which is placed to ease expiration by the wearer; this makes wearing the mask more comfortable but eliminates its ability for source control.
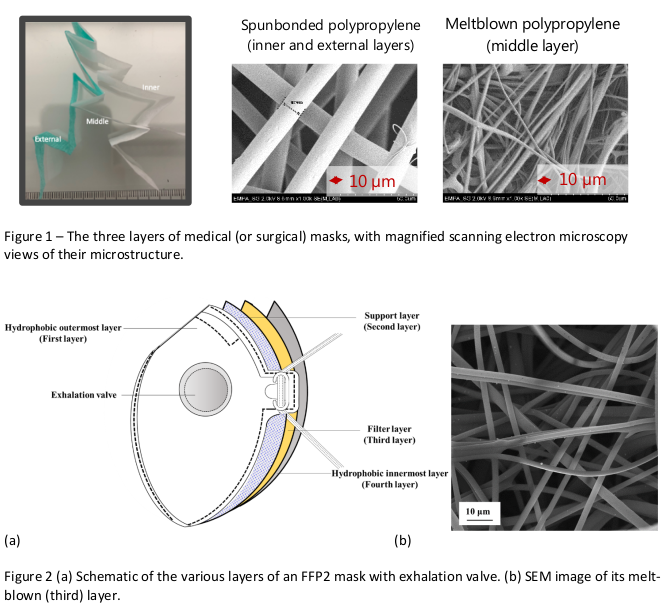
The blown polypropylene fiber main filtering layer is electrostatically charged, which further blocks the passage of very fine particles including virions. In medical masks the main filtering layer is generally fixed to two other layers, one on either side; those layers are also designed to serve functions that increase the performance of the mask. The inner (generally white and touching the wearer’s face) layer is hydrophylic, meaning it is water-absorbent, so as to block and keep within the mask droplets produced by the wearer. The outer (generally blue or green) layer is often hydrophobic, meaning that it is water-repellent, so that it prevents droplets from the outside (and also, in Type IIR masks, incoming high-velocity splashes) from penetrating the mask. Figure 1 details the three-layered structure of a commercially available medical mask. The structure of FFP-type protection masks is more complex although particle filtering also generally relies on a layer of meltblown polypropelyne fibers; Fig. 2.
Over time, the outer and central layers of protection- and medical masks as well as the outer surface of community masks, will collect all that may have been blocked while wearing the mask. The outer layer is potentially contaminated and thus, a source for contamination itself, particularly by touching. The inner layer contains the flora of the wearer and should not be touched by anybody else. Thus, removing and storing of used masks must take such considerations into account to avoid self-contamination and cross-contamination of others: using single use storage bags; performing hand hygiene after handling.
It is also important to realize that any hole, crack or tear in the mask will create breeches by which undesired particles can pass. Mechanical damage to the mask can reduce mask performance. It is therefore important to handle masks with care by avoiding piercing, tearing or even folding.
Those two factors, if well considered and managed, dictate most processes that underlie the proper use of masks for both source control and protection of the wearer. As one among many examples, folding a used mask in four and storing it in a pocket with its outer surface touching hands or clothes is clearly a bad idea. Similarly, touching the mask to adjust it while wearing it in public and then touching the eye or nose carries the risk of transfering infectious particles from the mask to wearer. In general, common sense, coupled with knowledge that the masks must not be mechanically damaged and that they will contain infectious particles should guide wearing, storing and disposing.
Considerations regarding sustainablitiy and the environment
Cleaning and decontamination of medical and protection masks
The shortage of masks during the first crisis of the COVID-19 pandemic highlighted the need for procedures, at least temporary, to extend their lifespan by reprocessing.
Legally, the re-use of an approved single-use protection or surgical mask, other than for personal use, is similar to the launch of a new product. It would only be possible in the case of serious shortage of protective equipment. An employer who wishes to recycle and redistribute FFP masks or equivalent to his employees will therefore only be able to do so (outside of a shortage situation) once it has been demonstrated that the masks, after decontamination, still have performances corresponding to their certification..
For FFP masks, as well as for surgical masks (used in healthcare environments), reprocessing must absolutely be carried out using protocols that have demonstrated their effectiveness in terms of decontamination without damaging the masks or reducing their efficacy (protection against aerosols in particular). For FFP masks, a few decontamination methods have been validated or are in the process of being validated by the Swiss COVID-19 task force [1]. The re-use of medical masks (outside healthcare environments) has the same legal constraints as the recycling of FFP mask or equivalent. Since surgical masks do not have any requirements in terms of aerosol penetration, it is likely that a number of decontamination or sterilisation procedures can be used. However, these procedures have yet to be demonstrated. .
Community type masks should be cleaned and recycled according to the manufacturer’s recommendations. As a general rule, machine washing with a regular detergent and at 60°C is sufficient. The number of cleaning cycles may vary depending on the material (at least 5 cycles for a community mask following the recommnadations of the Task Force ). In any case, the mask should be discarded if it is deformed or if the attachments are damaged (poor fit to the face).
Recommendations of the Science Taskforce
During the shortage of face masks at the height of the first wave of the COVID-19 pandemic, Swiss small and medium-sized entreprises (SMEs) and the Swiss Federation via the Krisenstab were looking for alternative strategies to address supply challenges. Textile-based facemasks were prototyped in collaboration; however, no definition of quality attributes for such masks were available. Therefore, the Swiss National COVID-19 Science Task Force provided, early in the crisis, recommendations defining community mask specifications in collaboration with a consortium of experts that had previously and informally assembled at the inception of the crisis (called the reMask consortium, https://www.remask.ch/). The purpose of the recommendations was to guide the industry in the development of textile masks of high quality for source control and some efficacy for protection, and they were based on current scientific knowledge on the subject (see also updated Policy Brief Recommendations for minimal specifcations for the community masks for Swiss manufacturers dated 24.9.2020).
As of September 2020, the Swiss National COVID-19 Science Task Force recommends the use of community masks meeting the recommendations outlined in the updated policy brief of September 2020 for source control in the greater public. Non-medical face masks that have not been tested against the recommendations or do not pass them, are not recommended, as they offer no garantee of adequate protection.
Beyond community masks, the Swiss National COVID-19 Science Task Force recommendations are in line with the WHO guidelines (FFP masks for aerosol-generating procedures, surgical masks for droplet precaution).
Situation on the availability and quality of face masks as per 31.8.2020
Next steps in Europe / Harmonization
At the height of the first wave of the COVID-19 pandemic, the Swiss National COVID-19 Science Task Force reacted to the shortage of face masks by providing expert consensus on minimal quality requirements for textile masks, as did similar initiatives in other countries. The consensus was based on (sparse) available information and consultation with specialists from various fields of expertise, and in liaison with task forces from other countries. Today, two guidance documents are available in Switzerland for reuseable textile face masks: one developed by the CEN Worshop (European Committee for Standardization) and directed by AFNOR (France), and another (differing only slightly) from the Swiss National COVID-19 Science Task Force (See Table 2). A number of countries have released similar guidance documents. As mentioned above, none of the documents are mandatory or legally binding.
All recommendations are consensus documents, which need to be checked against the day-by-day reality of production, use and reducing cross-transmission in the greater public. The future course of the COVID-19 epidemiology will provide more solid information to drive decision-making and harmonisation between the various stakeholders such as industry, regulators, scientists, and countries. Since there is a public interest to move towards a European technical standard, this process should be done within the frame of CEN or ISO.
Open scientific questions / next steps:
Several interdisciplinary consortia are funded by InnoSuisse or SNF NRP 78to:
- Optimize breathability, filtration efficacy and face fit
- Assess the durability and effects of regeneration methods on mask performance
- Develop and integrate detection and sensing systems for airborne viruses
- Compare the efficacy of wearing surgical masks and FFP2 masks during COVID-19 patient care
- Improve antiviral efficacy of textile masks
Safety and sustainability of textile facemasks compared to disposable masks are also addressed scientifically to build a sound knowledge base for the next generation of face masks.
Hygiene mask:
The expression hygiene mask is frequently used in the debate of face masks. However, the expression is not clearly defined for any particular type of face mask (is used for community as well as surgical masks) and therefore creates misunderstanding. We recommend differentiating between the three above categories, namely ultrafiltrating mask, surgical/medial mask and community mask.
Recommendation:
Recommendations can be issued by any body, expert or not, and are not legally binding. The validity and acceptance level of recommendations depends on the issuing organization / expert group and whether they are perceived by authorities and/or the general public as being credible. Although recommendations as such are never legal documents, national health authorities can declare them mandatory, which then makes them legally binding in given jurisdictions.
Technical standard:
A technical standard is an agreement on specific quality attributes of a product, assuring a defined and measurable (and hence certifiable) quality level. Technical standards are developed according to an established process and published by a consortium of laboratories, manufacturers and public authorities, generally across at least an entire country, or a larger international community. A technical standard requires harmonization and agreement on critical quality attributes, which can be tested by certified test laboratories or companies.
Test laboratories / companies:
Accredited institutions that test and provide test reports on the quality of a product according to technical standards or given norms (not to be confounded with notified bodies to issue certificates). In Switzerland we have several organisations qualified to test face masks.(*note by Andreas: should this not be community masks instead of face masks ?)
European Standard (EN):
European norms (EN) are standards and rules, which are approved by the European Commitee for Standardization (CEN). A new EN can be proposed by any member of the CEN (Switzerland is a member of CEN and Swiss experts can participate in the development of EN via SNV (Schweizerische Normen-Vereinigung). After workshop-based, cross-country agreement, a technical commitee will be mandated to develop an EN draft and share this with the CEN members until consensus is achieved. Thereafter, the draft will be formally accepted as an EN and will be re-evaluated every 5 years.
Notified body [8]:
A notified body, in the European Union, is an independent accredidited organisation that has been designated by a member state to assess the conformity of certain products, before being placed on the E.U. market, with the applicable essential technical requirements. These essential requirements are publicised in European directives or regulations. A manufacturer can use voluntarily European harmonised standards to demonstrate that a product complies with some (or all) of the EU essential requirements; a notified body can use the same harmonised standards to assess the conformity to these essential requirements. Conformity assessment can include inspection and examination of a product, its design, and the manufacturing environment and processes associated with it.
Certification:
Document confirming passing of all necessary tests (according the well-defined norms) giving the right to label and market the product accordingly. Certification is made by a notified body.
Market surveillance:
The purpose of market surveillance is to ensure the reliability and accuracy of the product information and the presence of the declared product characteristics and to ensure that products made available on the market do not present a risk to users.
Swiss stakeholders in the field of mask quality (non-prioritized and non complete list):
Schweizerischer Normenverband, ArmaSuisse, Testing labs, like Testex or SQTS, FOPH, Swissmedic, SECO, SUVA, BfU,
- https://e7b930e2-07a6-4ec5-a1a1-
b13908aa7993.filesusr.com/ugd/bcd50a_a67720ce464b416f9a56c0ae60de18c6.pdf - https://www.sixthtone.com/news/1005781/under-covid-19%2C-chinas-mask-market-surged.-now-its-gone-bust.
- https://www.deccanchronicle.com/world/asia/260420/china-confiscates-over-89-million-poor-quality-face-masks.html
- https://www.suva.ch/de-ch/die-suva/news-und-medien/medien/2020/07/17/ffp-schutzmasken-mit-mangeln
- https://www.swissmedic.ch/swissmedic/de/home/medizinprodukte/uebersicht-medizinprodukte/infos-zu-bestimmten-medizinprodukten/nicht_konformen_medizinischen_gesichtsmasken.html
- https://www.eu-esf.org/covid-19/4513-covid-19-suspicious-certificates-for-ppe
- https://www.swissmedic.ch/swissmedic/en/home/medical-devices/overview-medical-devices/information-on-specific-medical-devices/nicht_konformen_medizinischen_gesichtsmasken.html
- https://en.wikipedia.org/wiki/Notified_body
- https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/qualitaetsmaengel-und-chargenrueckrufe/chargenrueckrufe.html
Policy brief of the Science Taskforce on community mask spec and recommendations: https://ncs-tf.ch/de/policy-briefs/community-mask-spec-and-recommendations-25-april-20-en-2/download
Policy brief of the Science Taskforce on community mask spec and recommendations update Sept 2020
French guideline for community masks: https://masques-barrieres.afnor.org/
European specification for community masks:
https://www.cencenelec.eu/news/press_releases/Pages/PR-2020-004.aspx
Date of request: August/2020
Date of response: 14/10/2020
In response to request from: This statement was written in response to many queries from journalists, the population and procurement offices about mask types, quality and standards
Comment on planned updates: none
Expert groups and individuals involved: Infection Prevention and Control in collaboration with the reMask consortium ( https://www.remask.ch/ )
Contact persons: P. Wick (peter.wick@empa.ch), R. Rossi (rene.rossi@empa.ch)
Contributors: S. Tschudin Sutter, A. Mortensen, D. Jordi, W. Zingg, J.R. Delaloye, D. Decourten, D. Vernez