14 October 2020 – Policy Brief
Summary of request/problem
On request of the Swiss governmental Krisenstab, the “ReMask” Expert group formulated a recommendation for test methods and minimal specifications applicable to community masks. On 22.04.2020 these minimal specifications have been discussed and agreed upon with the Krisenstab and the Task Force VBS.
This document complements the document dated 25.4.20. It now additionally contains recommendations on handling and on the washing procedure applicable to community masks.
Executive Summary
Recommended specifications for community masks:
Community masks , mostly aimed at source control (meaning protection of others from the wearer), should offer a sufficient protection against liquid droplets of different sizes and aerosols (particle size down to 1 micrometer) produced by the wearer, for example while talking, coughing or sneezing. Community masks should have a sufficient air permeability to minimize breathing hindrance and shoud be available in different fitting sizes appropriate for both adults and children, so as to guarantee an adequate face coverage.
In brief, the following minimal specifications are recommended:
- Air permeability < 60 Pa/cm2 according to ISO 9237
- Splash resistance: no liquid penetration as defined in EN 14683:2019+AC:2019
- Mask filtration efficiency FE ≥ 70 % with a particle size of 1 micrometer.
- >5 times washable at 60 degree Celsius with usual detergent
Recommendation on handling and washing frequency of community masks
Main text
Definition of the Community masks specification and their testing procedure
Mask terminology
- FFP Masks, Filtering Facepiece Particles facemasks, or personal protection facemasks are masks meeting the criteria of the norm EN 149 (e.g. FFP1 -3, , N95, KN 95 or equivalent) FFP masks are personal protective equipment and have to comply with the PPE-directive (EU/2016/425, SR 930.115 – Verordnung über die Sicherheit von persönlichen Schutzausrüstungen (PSA-Verordnung)). They have to be tested according to the European Standard EN 149 and certified by an accreditated Notify Body (before they can be put on the market). FFP masks are classified into FFP1, FFP2 and FFP3 depending on their filtration ef-ficiency.FFP Masks, Filtering Facepiece Particles facemasks, or personal protection facemasks are masks meeting the criteria of the norm EN 149 (e.g. FFP1 -3, , N95, KN 95 or equivalent) FFP masks are personal protective equipment and have to comply with the PPE-directive (EU/2016/425, SR 930.115 – Verordnung über die Sicherheit von persönlichen Schutzausrüstungen (PSA-Verordnung)). They have to be tested according to the European Standard EN 149 and certified by an accreditated Notify Body (before they can be put on the market). FFP masks are classified into FFP1, FFP2 and FFP3 depending on their filtration ef-ficiency.
- Surgical Masks, OP-Masks, or Medical masks are masks meeting the criteria of the norm EN 14683 (e.g. Type I, Type II, Type IIR, or equivalent) Surgical masks have to comply with the regulation on medical products (EU/2017/745, SR 812.213 Medizinprodukteverordnung – MepV). They have to meet the requirements of European Standard EN 14683 but do not require third party assessment(before they can be put on the market). There is hence no ‘certification’ as such and compliance is demonstrated by the manufacturer Surgical masks are classified into Type I, Type II and Type IIR. Only Type IIR offers a protection against liquid splashes.
- “Community” mask is not an official term; it is used here for masks that don’t need to meet the requirements of European Standard EN 14683 or EN 149. The use of such non-certified community masks is aimed at the general population, primarily for source control (respiratory etiquette) – thus, to protect others from exhaled virus-containing droplets or aerosols emitted by the mask bearer. Not all mask designs or materials are suitable for community masks and research is presently being conducted to identify the best mask designs. Performance criteria defining masks that are sufficiently blocking to droplets while being comfortable to wear and allowing reprocessing by cleaning do not correspond to an existing standard. As a result, this new specification list is defined and justified in the present document and is a recommendation.
Splash/droplet resistance
This test is defined in ISO 22609:2004. It is performed for new masks as well as reprocessed masks after a number of wash-/ decontamination cycles but below the maximum number guaranteed by the manufacturer. The test method is in line with ISO 22609:2004 and it is intended to evaluate the protection of the person wearing the mask from exposure to blood and other body fluids. The test evaluates the resistance of masks to penetration by a fixed volume of synthetic blood applied to the mask by high-velocity liquid contact over a time period between 0 s and 2,5 s. Outcome is based on visual and/or optical inspection of synthetic blood penetration.
In detail, the test method consists of spraying/squirting a volume of liquid horizontally onto the mask at a defined speed, corresponding to human blood pressure (16 kPa in EN 14683), in order to simulate the scenario of a mask being contaminated by a punctured blood vessel. This pressure is higher than what is known to occur during sneezing (7 kPa [2]) and also higher than the maximal static expiratory mouth pressure (13 kPa [3]). After projecting the synthetic blood on the outside,the internal side of the mask is inspected for penetration of liquid. A swab can be used to test the target area in case of doubt of visual inspection.
The droplet size distribution during coughing is summarised in Figure 1 [4]. The droplet size is determining the resistence time in the air. However the debate is still ongoing and partially addressed in previous Policy Briefs of the National COVID-19 Science Task Force [12,13, 14]
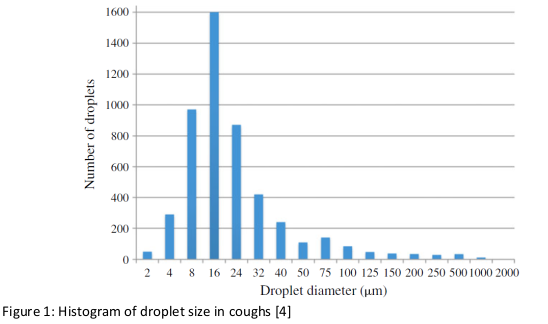
In order to simulate coughing more precisely than does a blood jet, a synthetic colored artificial saliva (according to [7]) is used with a pressure of 12 kPa. The saliva mass is 2.04 ±0.040 g. The masks are preconditioned 4 hours at 21°C and 85% rH.
Minimal requirement: no liquid penetration in 10 specimens
Aerosol filtration efficiency
This test is intended to evaluate the filtration efficiency and thus protection of the person wearing the mask from exposure to aerosols or droplets. Studies on the exhaled breath of influenza infected patients contained about 70% of influenza virus in particles between 300 nm and 500nm [5]. FDA-cleared surgical mask models tested varied between 7.5 – 76.3% filtration efficacy at 85 liters/minute with particles ranging from 40nm-1000nm [6]. Therefore, filtration efficiency is determined by exposing the mask to aerosol particles (particle size: 1 µm) applying laminar flow.
Minimal requirement: Mask filtration efficiency FE ≥ 70 % with particle size of 1 µm.
Air permeability
The mask must have sufficient air permeability to allow normal breathing.
Minimal requirement: according to EN 14683, the pressure difference must be <60 Pa/cm2 during a test of air permeability, applied according to the norm ISO 9237: 1995 (Textiles – Determination of the permeability of fabrics to air). Measurements should be carried out at a pressure differential of 100 Pa and a test area of 4.9 cm2.
Innocuity of the materials
Materials in contact with skin must not be irritative or toxic, e.g. they must comply with ISO 10993-1:2018 (Biological evaluation of medical devices – Part 1: Evaluation and testing within a risk management process). If chemicals are used (for hydrophobicity, virucial effect, etc.); masks also must comply with the REACH regulation.
Handling
We recommend washing the community masks once per day if worn twice daily (e.g. using it in public transportation in the morning and evening). In the meanwhile, while not worn, the community mask should be stored in a way that avoids cross contamination (from the potentially contaminated outer surface to the clean inner surface) and avoids damaging the integrity of the mask (in terms of loss of function of the textile and poorer fit):
- Storing the mask unfolded within a disposable, breathable bag (e.g.paper envelope, after use to be disposed – or textile bag, to be washed with the mask) so that the outer surface of the mask always faces the same direction within the bag, thus avoiding cross-contamination of the inner mask surface is most favorable. Plastic bags, that will keep humidity, should be avoided due to an increased risk of bacterial or fungal overgrowth in the mask.
- Folding the mask may damage the textile or the metal piece negatively affecting mask performance or the fit of the mask. On the other hand folding of the mask may reduce the risk of cross contamination within a storage bag if the mask is folded so that the outer surface is hold inward and against itself to reduce contact with inner surface during storage. For most mask designs a horizontal fold may be less damaging than a vertical fold as the metal piece is not affected by the fold.
- As the outer surface of the mask may have become contaminated by use, hand hygiene should always be performed before and after touching the mask.
Washing procedure and reusability
Masks, including textiles and straps, must tolerate at least 5 washing cycles at 60°C in a domestic washing machine (Washmaschine Type A) with phosphate-free detergents (‘Pulvervollwaschmittel’) including a dry programme according to European Standard DIN EN ISO 6330 and must do so without loss of barrier properties or degradation of the elastic material.
The manufacturer should provide an easy method to allow one to control of the number of washing cycles (e.g. knots in the straps, waterproof marking, etc.). Masks should be disposed after the maximum number of washing cycles has been achieved.
Washing masks with detergent at 60°C is currently the only officially recommended method. However, alternative methods like 30°C washing [9, 10], heat treatment by a household oven at 70°C for 30 min [11] or ironing have been proposed by other expert groups.
Mask design
The mask must be designed to cover nose, mouth and chin and it should guarantee close fitting on the sides. Different sizes should be produced to allow appropriate and safe use in different populations (children, adults). Anthropometric details can be found in ISO/TS 16976-2:2015 (Respiratory protective devices – Human factors – Part 2: Anthropometrics).
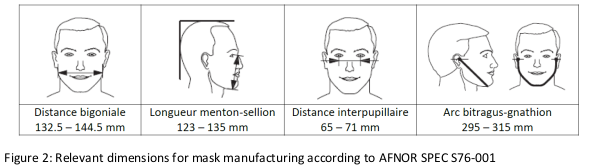
Figure 2 shows the specifications of the French mask task force (AFNOR SPEC S76-001) [8] for adults.
Wear comfort
Straps must allow easy donning and doffing of the mask. They must be strong enough to maintain the mask in place during use, and sufficiently elastic to allow easy fit. They must maintain elasticity after repeated use and particularly after washing.
Unresolved issues
Futher investigations regarding the efficacy of masks for non-healthcare professionals is needed. New data acquired during the pandemic will be monitored and revisions will be made continuously as required.
- https://www.afnor.org/en/news/protective-masks-faced-with-coronavirus-standard-development-bodies-follow-multiple-leads/, accessed 11 April 2020.
- Rahiminejad, M., Haghighi, A., Dastan, A., Abouali, O., Farid, M., & Ahmadi, G. (2016). Computer simulations of pressure and velocity fields in a human upper airway during sneezing. Computers in biology and medicine, 71, 115-127.
- Man, William DC, et al. “Cough gastric pressure and maximum expiratory mouth pressure in humans.” American journal of respiratory and critical care medicine 168.6 (2003): 714-717.
- Bourouiba, Lydia, Eline Dehandschoewercker, and John WM Bush. “Violent expiratory events: on coughing and sneezing.” Journal of Fluid Mechanics 745 (2014): 537-563.
- Fabian P, et al. ” Influenua Virus in Human Exhaled Breath: an observational study” Plos One July 2008 3(7)e2691
- Rengasamy S et al ”Filteration Performance of FDA-Cleared surgical masks” J Int Soc Respir Prot. 2009 26(3):54-70
- Łysik, Dawid, et al. “Artificial saliva: Challenges and future perspectives for the treatment of xerostomia.” International journal of molecular sciences 20.13 (2019): 3199.
- https://masques-barrieres.afnor.org/, accessed 11 April 2020.
- http://www.academie-medecine.fr/communique-de-lacademie-du-bon-usage-des-masques/
- Gerhardts A, et al. ”Testing of the Adhesion of Herpes Simplex Virus on textile subtratesand its inactivation by household laundry processes, J Bioscience and Medicine2016(4)111-125
- https://www.ruhr24.de/service/mundschutz-coronavirus-maske-waschen-viren-reinigen-tipps-corona-maskenpflicht-verbraucher-nrw-13697617.html
- Policy Brief: Benefits of wearing mask in community settings where social distancing cannot be reliably achieved from 1.7.2020
- Importance of seasonality and climate on the risk of COVID-19 (26 May 20-EN) published 02. 06. 2020 [14] Policy Brief: Responses to specific FOPH, June 3 2020
Date of request: –
Date of response: 14/10/2020
In response to request from: update on handling and the washing procedure for community masks
Comment on planned updates: none planned as of writing
Expert groups and individuals involved: Sub task IPC; S. Tschudin Sutter, P. Wick, R. Rossi, A. Mortensen and ReMask Expert group https://www.remask.ch/
Contact persons: Peter Wick (peter.wick@empa.ch), René Rossi (rene.rossi@empa.ch)