23 June 2020 – Policy Brief
Summary of request/problem
During the peak of the COVID-19 pandemic in Switzerland, procurement of certified and properly tested face masks (FFP masks as well as surgical masks) has been a challenge. Similar to other countries, low quality face masks have flooded the Swiss market and although the official regulations on certification and testing are clear, there is concern that some procurers might not be aware of them. This paper aims to help actors in the Swiss supply chain face this crisis situation and gives information 1) to list applicable regulations and processes for face mask procurement that should be followed, 2) to summarise relevant policies and theirexceptions, and 3) to help identify doubtful face masks by examination of the documentation made available.
Executive summary
During the peak of the COVID-19 pandemic in Switzerland, procurement of certified and properly tested face masks, including filtering facepiece (FFP) and surgical masks, became difficult. Further into the pandemic, the Swiss market was flooded with products for which legitimate concerns regarding the accuracy of disclosed certificates have arisen. To protect the Swiss health system, other professional personnel categories and the wider public during this time, different laboratories across Switzerland have implemented simplified qualification tests on procurable face masks with the aim to assess whether those masks obeyed minimum protection standards. Unfortunately, it was found that low quality products are being sold on the Swiss market, putting pressure on both users and procurers. Procuring face masks, both FFP and surgical, should be brought back to the normal (pre-COVID-19) state where only certified products are put on the market and both procurers and users reliably follow the regulations issued by Swissmedic and SECO.
Introduction
For the present discussion we distinguish between FFP and surgical masks (the latter being also called hygienic masks or medical masks) because the responsibility for the regulation of their procurement and use in Switzerland lies with different authorities.
FFP Masks
FFP masks also know as personal protection face masks must meet the requirements of European StandardEN 149 and are equivalent to N95 and KN95 which are certified in USA and China, respectively. FFP masks are personal protective equipment (PPE) and have to comply with the PPE-directive (EU/2016/425, SR 930.115 – Verordnung 2 über die Sicherheit von persönlichen Schutzausrüstungen (PSA-Verordnung)). FFP masks have to be certified by an accreditated external agency, also called Notified Body (NB), and are classified into FFP1, FFP2 and FFP3 depending on their aerosol filtration efficiency.
Surgical Masks
Surgical masks, OP-masks, or medical masks must meet the requirements of European Standard EN 14683 and have to comply with the directive on medical products (EU/2017/745, SR 812.213 Medizinprodukteverordnung – MepV); however, they are classified as ‘Class I’ Medical Devices and as such do not require third party assessment. There is hence no ‘certification’ as such and compliance is demonstrated by the manufacturer.1 Surgical masks are classified into Type I or Type II, depending on their biological filtration efficiency. A Type IIR offers an additional protection against liquid splashes.
Swissmedic (surgical masks)
Swissmedic issues warning about non-conforming surgical masks (01.05.2020)2
The COVID-19 pandemic has dramatically increased the demand for surgical masks, in particular in Swiss healthcare institutions. By enquiries and receiving requests for special approval, Swissmedic has observed that operators with no previous experience in the sector of therapeutic products have been importing and selling surgical masks. This may have resulted in non-conforming, low-quality or even counterfeit products appearing on the Swiss market. Non-conforming or counterfeit surgical masks do not guarantee protection as defined by EN 14683.
Procurement of medical devices in healthcare institutions – checking medical devices
Swissmedic recommends that healthcare institutions should ignore promotional emails from dubious sources (spam) and procure conforming products through established procurement channels. It alsorecommends that buyers who are considering new suppliers should thoroughly inspect documents(particularly certificates), verify the source and supply chains of the masks on offer, and refer to the information in the appropriate Swissmedic information document.
The following documentation is important for surgical masks (Class I non-sterile medical devices):
- Declaration of conformity (DoC) in accordance with Directive 93/42/EEC (or Regulation (EU) 2017/745) by the manufacturer or its European authorised representative
- Details of the European authorised representative (EC-REP) on the packaging if the manufacturer is located outside Europe
- Details of the manufacturer on the product itself (this must be identical to the information in the DoC)
By signing the DoC the manufacturer or its authorized representative take full responsibility for their product’s compliance with the applicable EU law. Unfortunately, numerous misleading and counterfeit documents have been submitted as proof of conformity. To help identify them, the following list summarizes the main information that this DoC should contain:3
- Title: EU Declaration of Conformity
- A number identifying the product
- The name and address of the manufacturer or of the authorised representative issuing the declaration
- A statement that the declaration is issued under the sole responsibility of the manufacturer
- The identification of the product allowing traceability
- The relevant legislation with which the product complies, as well as any harmonised standards or other means used to prove compliance
- The name and identification number of the notified body when it has been involved in the conformity assessment procedure and the reference to the relevant certificate, if applicable
- Supplementary information, if applicable
- The date and place of issue of the declaration; signature
Note: The exemption for non-conforming surgical masks, asapproved by the Federal Council on 29 April 2020 in COVID-19 Ordinance 2, only applies to non-medical use. Such non-conforming masks must NOT be used for direct patient contact, neither in hospitals nor in other healthcare settings!
Report non-conforming medical devices
Purchasers of medical devices who notice irregularities (e.g. potentially counterfeit EC certificates) with a product, should report their suspicions to Swissmedic. Swissmedic checks such reports on the basis of the associated exposure risk, and takes corrective action, involving other European authorities where necessary.
Exemples of misleading and counterfeit documents are given on the Swissmedic website.
Swissmedic also offers an “Information sheet on procurement of medical devices in health institutions”.
SECO (FFP masks)
SECO regulates the use of FFP masks in Switzerland.4
Normally, this product should respect the rules and procedures for the conformity assessment under Article 3 paragraph 2 of the PPE Ordinance of 25 October 2017 (PPEO).5 However, due to their high demand and supply difficulties induced by COVID 19 pandemics, derogations have been published on March 13 to facilitate their availability and to combat the spread of the coronavirus in our country (Ordinance on Measures to combat the coronavirus).6 In this situation, the procedure for the assessment of the conformity of the FFP mask in the sense of Art. 3, para. 2, PPEO is not anymore necessary, although the mask still mustprovide its user with a level of security comparable to the requirements of the PPEO.
Derogations for the release of FFP masks on the Swiss market during COVID 19 pandemics are listed below:7
- The FFP mask may have been manufactured in accordance with an harmonised European standard, but the conformity assessment procedure has not been carried out or is still pending.
- The FFP mask may have been manufactured in accordance with a standard that is cited in the WHO guidelines but is not a harmonized European standard.
- The FFP mask was manufactured according to a non-European standard, for example according to a Japanese standard, and can be placed on the market in Japan for similar use in accordance with the latter.
- The FFP mask was manufactured according to another technical solution, which yet has to be evaluated and approved by an inspection body. This approval may be granted on the basis of an accelerated type examination or other requirements.
SECO publishes on its website examples of applications for each of these exceptions (see below).4 However, besides derogation number 2, the manufacturer or importer must inform SECO and send a document to coronavirus@seco.admin.ch containing the following information:
- Proof from a laboratory that certifies at least the application of the testing principle of the respiratory protection mask against SARS-Cov-2 pandemic elaborated by the Zentralstelle der Länder für Sicherheitstechnik (ZLS)8
- Copy of product marking
- Product image
- Product and type description
- Name and address of the manufacturer
- Number of products that are expected to be placed on the market under this exception.
As for surgical masks, suspicious certificates are currently being presented as proof of compliance of the FFP mask.9 In this situation, we recommend carefully analyzing certicates for FFP-type masks using the following criteria:
- Control of the number and the name of the NB: Official NBsaccredited to test FFP respirators masksare listed in the NANDO (New Approach Notified and Designated Organisations) Information System.10
- Checking the list of laboratories accredited in China for testing of KN95 masks: on the face of a Chinese certificate, the certificate number and the name of the laboratory should be recorded in the list of laboratories accredited for testing KN95 masks edited by the China National Accreditation Service for Conformity Assessment (CNAS).11
- Conformity assessment results in a document entitled ‘EU type examination certificate’.9 Any other certificate could be authentic but has no value:12
- A Certificate Of Compliance is issued on a purely voluntary basis and is not a CE Certificate. Therefore, it cannot replace a correct EU declaration of Conformity.
- A Certificate of FDA Registration is just a registration number for US trade; it does not imply approval of the facility or of its products.
Finally, we also recommend identifying printing errors on the external side of FFP mask as information written on it are is strictly regulated; this is are summarized in Table 1.
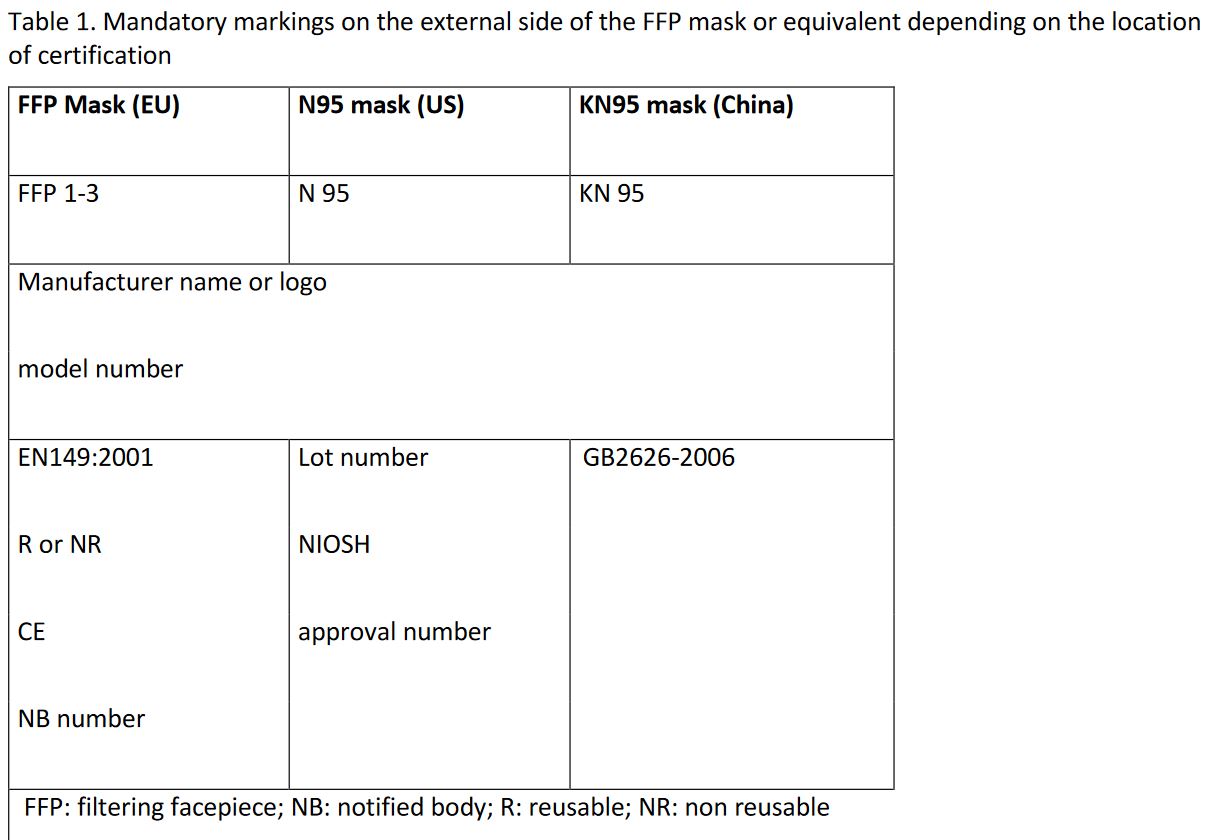
In their FAQ document on regulations and exceptions for marketing FFP masks in Switzerland, SECO addresses the main conditions as follows:
Was für Atemschutzmasken dürfen in Verkehr gebracht werden?
Unter den Voraussetzungen von Artikel 4o COVID-19-Verordnung 2 dürfen Atemschutzmasken in Verkehr gebracht werden, die ein angemessenes Sicherheitsniveau gemäss den Anforderungen der schweizerischen PSA-Verordnung (SR 930.115) gewährleisten.
Welche Möglichkeiten ergeben sich, eine Atemschutzmaske nach der Sonderregelung gemäss Artikel 4o COVID-19-Verordnung 2 in Verkehr zu bringen?
In Artikel 4o Absatz 2 COVID-19-Verordnung 2 sind folgende Ausnahmen abgebildet:
Erste Ausnahme (Artikel 4o, Absatz 2, Buchstabe a)
Die Atemschutzmaske gewährleistet ein angemessenes Sicherheitsniveau im Hinblick auf die geltenden rechtlichen Anforderungen und die Herstellung erfolgt nach einer harmonisierten europäischen Norm mit ausstehendem Konformitätsbewertungsverfahren.
Für die Freigabe einer Atemschutzmaske, welche nach dieser Ausnahme in Verkehr gebracht werden soll, benötigt das zuständige Kontrollorgan einen Nachweis einer benannten Stelle, dass die Atemschutzmaske den im Auftrag der deutschen Zentralstelle der Länder für Sicherheitstechnik (ZLS) entwickelten Prüfgrundsatz (Rev. 2 – 02.06.2020) für Corona SARS-Cov-2 Pandemie Atemschutzmasken (ZLS CPA – Rev. 2 – 02.06.2020)erfüllt.
Dem Antrag sind beizulegen:
- Baumusterprüfbescheinigung (ZLS CPA – Rev. 2 – 02.06.2020) der benannten Stelle,•Kopie der Kennzeichnung des Produktes,
- Abbildung des Produktes,
- Typen- und Produktebezeichnung,
- Name und Adresse des Herstellers,
- Anzahl Produkte, die geplant sind, nach dieser Ausnahme in Verkehr zu bringen.
Der Antrag mit den genannten Unterlagen ist an coronavirus@seco.admin.ch einzureichen. Das SECO leitet den Antrag an das zuständige Kontrollorgan zur Überprüfung weiter.
Zweite Ausnahme (Artikel 4o, Absatz 2, Buchstabe b)
Die Atemschutzmaske gewährleistet ein angemessenes Sicherheitsniveau im Hinblick auf die geltenden rechtlichen Anforderungen und die Herstellung erfolgt nach einer in den WHO-Richtlinien genannten Norm.
Eine Atemschutzmaske, welche dem NIOSH (National Institute for Occupational Safety and Health-Standard) N95 entsprechend hergestellt und von NIOSH zertifiziert ist, darf ohne weitere Prüfung durch das Kontrollorgan in der Schweiz in Verkehr gebracht werden. Im Falle einer Kontrolle muss vom Inverkehrbringer der entsprechende Nachweis erbracht werden können. Alle von der NIOSH zertifizierten Masken sind in der Certified Equipment List aufgeführt.
Dritte Ausnahme (Artikel 4o, Absatz 2, Buchstabe c, erste Möglichkeit)
Die Atemschutzmaske gewährleistet ein angemessenes Sicherheitsniveau im Hinblick auf die geltenden rechtlichen Anforderungen und die Herstellung erfolgt nach einer nicht-europäischen Norm.
Für die Freigabe einer Atemschutzmaske, welche nach dieser Ausnahme in Verkehr gebracht werden soll und welche in einem nicht-europäischen Markt verkehrsfähig ist, benötigt das Kontrollorgan folgende Nachweise, die dem Antrag beizulegen sind:
- Baumusterprüfbescheinigung (ZLS CPA – Rev. 2 – 02.06.2020) eines von einer nationalen Behörde akkreditierten Prüflabors,
- Baumusterprüfbericht des Prüflabors,
- Kopie der Kennzeichnung des Produktes,
- Abbildung des Produktes,
- Typen-und Produktebezeichnung,
- Name und Adresse des Herstellers,
- Anzahl Produkte, die geplant sind, nach dieser Ausnahme in Verkehr zu bringen.
Der Antrag mit den genannten Unterlagen ist an coronavirus@seco.admin.ch einzureichen. Das SECO leitet den Antrag an das zuständige Kontrollorgan zur Überprüfung weiter.
Vierte Ausnahme (Artikel 4o, Absatz 2, Buchstabe c, zweite Möglichkeit)
Die Atemschutzmaske gewährleistet ein angemessenes Sicherheitsniveau im Hinblick auf die geltenden rechtlichen Anforderungen und die Herstellung erfolgt nach einer anderen technischen Lösung.
Für die Freigabe einer Atemschutzmaske, welche nach dieser Ausnahme in Verkehr gebracht werden soll, benötigt das zuständige Kontrollorgan einen Nachweis eines vom SECO anerkannten Prüflabors, dass das Produkt mindestens den ZLS CPA – Rev. 2 – 02.06.202 erfüllt.
Dem Antrag sind beizulegen:
- Baumusterprüfbescheinigung (ZLS CPA – Rev. 2 – 02.06.2020) des Prüflabors,
- Baumusterprüfbericht des Prüflabors,
- Kopie der Kennzeichnung des Produktes,
- Abbildung des Produktes,
- Typen- und Produktebezeichnung,
- Name und Adresse des Herstellers,
- Anzahl Produkte, die geplant sind, nach dieser Ausnahme in Verkehr zu bringen.
Der Antrag mit den genannten Unterlagen ist an coronavirus@seco.admin.ch einzureichen. Das SECO leitet den Antrag an das zuständige Kontrollorgan zur Überprüfung weiter.
Non regularly procured products
All products must fulfill the above mentioned regulations or exemptions. Tests performed by a non-accredited laboratory do not exonerate the procurer from these regulations and exemptions.
Recommendation
In preparation for a possible future increase of cases of COVID-19, we strongly recommend that the relevant stakeholders in the Swiss healthcare and public health community use the time made available to build and stock their supplies with material of good quality meeting all relevant standards and regulations, according to the pandemic plan. If any non-conforming product remains in stock and is aimed to be used in the future,it must be remembered that the regulations of Swissmedic and SECO outlined above do apply. If no certificate of exemption has yet been issued by Swissmedic or SECO for the material in question, suppliers and purchasers are called to apply for such a certificate in due time.
Unresolved issues
-N/A-
[1] CE marking & COVID-19 – Update; Available from: https://www.cemarkingassociation.co.uk/author/cemarking/
[2] New coronavirus: Swissmedic issues warning about non-conforming face masks; Available from: https://www.swissmedic.ch/swissmedic/en/home/medical-devices/overview-medical-devices/information-on-specific-medical-devices/nicht_konformen_medizinischen_gesichtsmasken.html
[3] Technical documentation and EU declaration of conformity; Available from: https://europa.eu/youreurope/business/product-requirements/compliance/technical-documentation-conformity/index_en.htm
[4] FAQ Atemschutzmaske und andere PSA; Available from: https://www.seco.admin.ch/seco/de/home/Arbeit/Arbeitsbedingungen/Produktsicherheit/produktesicherheit_faq_covid19.html
[5] Verordnung über die Sicherheit von persönlichen Schutzausrüstungen; Available from: https://www.admin.ch/opc/fr/classified-compilation/20172047/index.html
[6] Ordinance on Measures to Combat the Coronavirus (COVID-19); Available from: https://www.admin.ch/opc/en/classified-compilation/20200744/index.html
[7] Erläuterungen zur Verordnung 2 über die Bekämpfung des Coronavirus/Rapport explicatif concernant l’ordonnance 2 du 13 mars 2020 sur les mesures destinées à lutter contre le coronavirus (ordonnance 2 COVID-19); Available from: https://www.bag.admin.ch/bag/fr/home/krankheiten/ausbrueche-epidemien-pandemien/aktuelle-ausbrueche-epidemien/novel-cov/massnahmen-des-bundes.html#-993003382
[8] Prüfgrundsatz für Corona SARS-Cov-2 Pandemie Atemschutzmasken; Available from: http://www.zls-muenchen.de/Corona/Atemschutzmasken/200602_Pruefgrundsatz%20Corona%20SARS-Cov-2%20Pandemie%20Atemschutzmasken%20Rev.%202.pdf
[9] suspicious certificates for PPE – updated 18/06/2020; Available from: https://www.eu-esf.org/covid-19/4513-covid-19-suspicious-certificates-for-ppe
[10] European Commission. Notified Body NANDO; Available from: https://ec.europa.eu/growth/tools-databases/nando/index.cfm?fuseaction=directive.notifiedbody&dir_id=155501.
[11] China National Accreditation Service for Conformity Assessment (CNAS). Laboratories Accredited by CNAS for Testing of Masks, Gloves, Medical Protective Clothing and Other Personal Protective Equipment. 2020, april 27; Available from: https://www.cnas.org.cn/english/photonews/06/903064.shtml
[12] Center for Disease Control and Prevention (CDC). Factors to consider when purchasing respirators from another country. 2020, may 7; Available from: https://www.youtube.com/watch?v=w7tVnjrmAmc.
Date of request: June/2020
Date of response: 23/06/2020
In response to request from: This statement was written in response to the results of quality tests of face masks
Comment on planned updates: none
Expert groups and individuals involved: Prevention and Control in collaboration with the reMask consortium ( https://www.remask.ch/
Contact persons: D. Jordi, JR. Delaloye, P. Wick, D. de Courten, S. Tschudin-Sutter
Contributors: D. Jordi, JR. Delaloye, P. Wick, D. de Courten, W. Zingg, S. Tschudin-Sutter